The average length of an orthodontic treatment varies between one and three years, depending on many factors, such as degree of the problem, the biotype and patient compliance. The big question is how can we shorten the treatment time without affecting the biology and the overall outcome of the case? Safely accelerating therapy enhances the motivation of adult patients, as time is a valuable commodity.
One of the methods used to speed up tooth movement is photo-biomodulation therapy. Photo-biomodulation has been described in the literature as a promising and effective therapy for accelerating orthodontic movement and improving its efficacy and comfort during orthodontic treatment, having an analgesic and bone remodelling effect.1,2
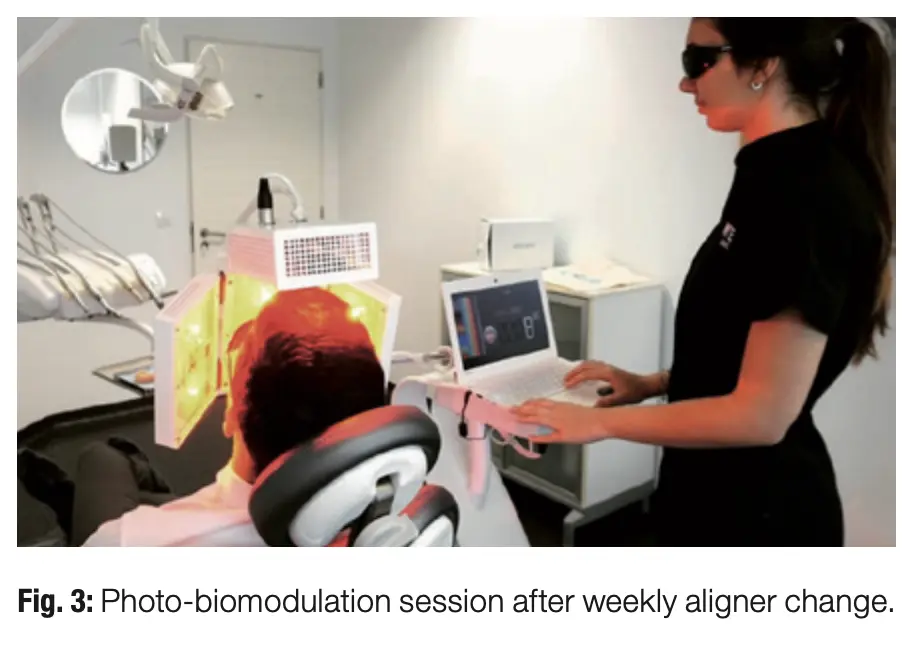
Also known as low-level light therapy, photo-biomodulation therapy has been demonstrated to be a minimally invasive technique that appears to be effective. The application of light of a specified set of wavelengths over an appropriate period has been shown to be a means to accelerate orthodontic movement. This technique has the objective of activating cells at a mitochondrial level to make them produce more energy, adenosine triphosphate, which is essential for cell repair and regeneration. There are several LED devices on the market, however, the ATP38 (Swiss Bio Inov) has proved to be the most efficient, since it employs semiconductor technology to focus the light emitted, creating an energy similar to that of a laser beam, rather than dispersing the light like other LED devices do.3
But how exactly can photo-biomodulation be good for orthodontic movement?
Firstly, we need to understand how orthodontic forces work and the biological process of tooth movement. The application of orthodontic (mechanical) forces initiates an inflammatory process in the periodontal complex and a cellular chain reaction that results in tooth movement. Orthodontic tooth movement is therefore the result of remodelling changes in the bone adjacent to the periodontal ligament, arising from the effects of bone resorption by osteoclasts in areas of pressure and bone deposition in areas of root tension. When bone resorption and deposition occur around different areas of the same tooth, the result is tooth movement.
The periodontal ligament is rich in cells and blood vessels. It is made up of undifferentiated stem cells, which have the potential to develop into osteoblasts, cementoblasts or fibroblasts. For bone remodelling, osteoblasts, osteoclasts and fibroblasts communicate via the RANKL–RANK–OPG signalling pathway, which involves the interaction of receptor activator of nuclear factor kappa B ligand (RANKL), receptor activator of nuclear factor kappa B (RANK) and osteoprotegerin (OPG).
The most abundant cell in the ligament is the fibroblast, which is responsible for its structure by producing collagen. It also secretes RANKL and transforming growth factor β (TGF-β) under mechanical stimulation. Stem cells differentiate into osteoblasts under the influence of the growth factor TGF-β. Osteoblasts (responsible for bone formation) secrete RANKL, OPG, macrophage colony-stimulating factor (M-CSF) and several other molecules.
Osteoclasts (responsible for bone resorption) differentiate into pre-osteoclasts from haematopoietic stem cells present in the vascular system. These pre-osteoclasts migrate to bone sites and fuse into osteoclasts in the presence of RANKL and M-CSF.
When mechanical force is applied, an acute inflammatory response is triggered, inducing vasodilation and al- lowing leucocytes to penetrate from the capillaries into the ligament matrix. Leucocytes produce cytokines, which initiate the signalling pathway. The osteoclasts remain active for as long as the adjacent cells are producing RANKL.
Under the influence of anabolic and hormonal (calcitonin, oestrogen), immunological (TGF, interleukin 17 and 4) or inorganic (calcium) factors, osteoblasts produce and secrete OPG. This molecule binds to RANKL, preventing it from attaching to its RANK transmembrane receptor and stopping osteoclastic activity. Osteoblasts therefore play a key role in bone remodelling, as they are involved in activating osteoclastogenesis, inhibiting reabsorption and synthesising new bone matrix. Thus, in areas of compression, RANKL levels are high, and in areas of tension, TGF-β and OPG levels are much higher.
Photo-biomodulation therapy can play a key role in orthodontic movement, since it has the effect of increasing cell proliferation, collagen synthesis and the release of growth factors and other cytokines that stimulate the activity of osteoblasts, osteoclasts and fibroblasts involved in bone remodelling.
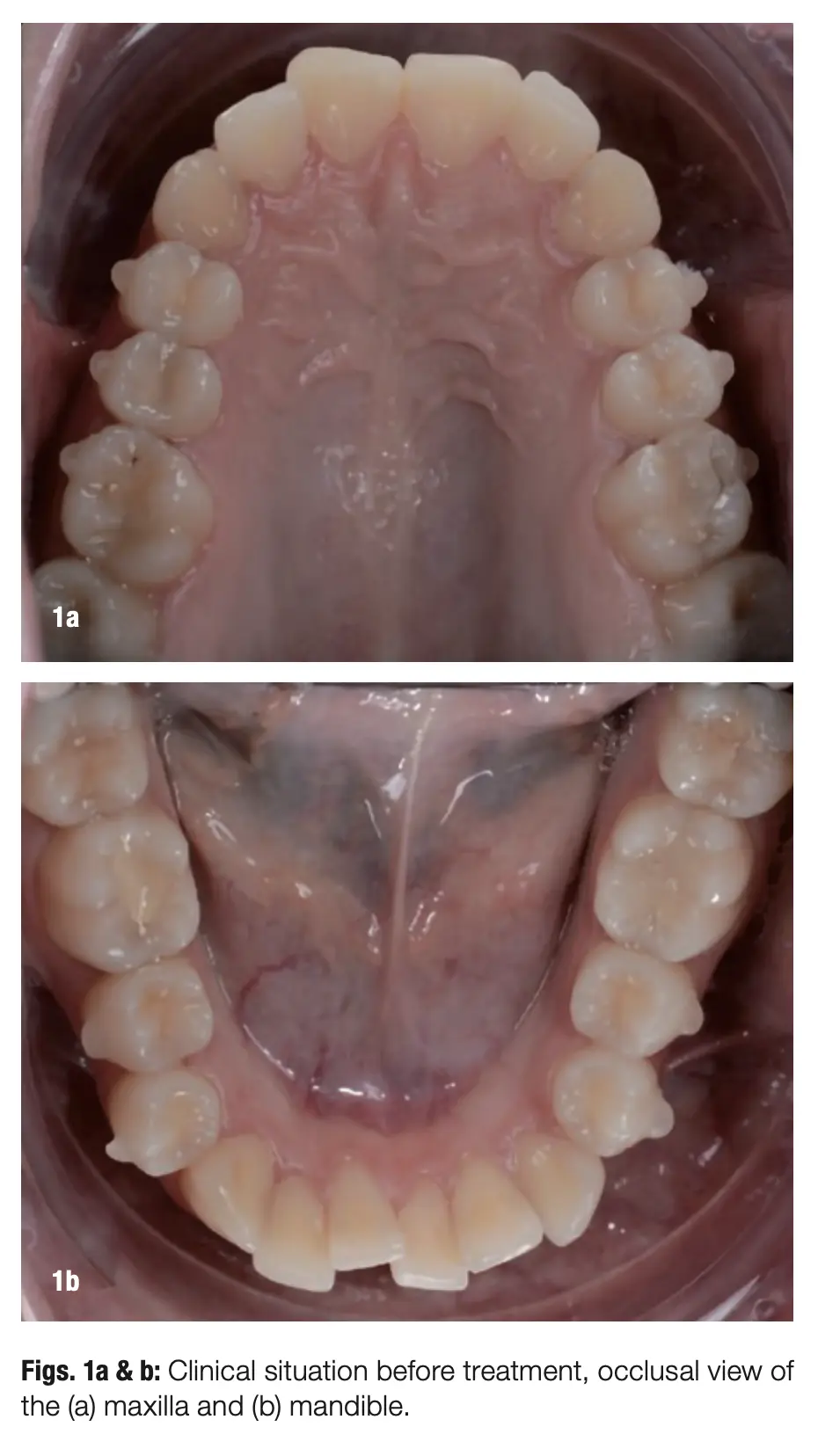
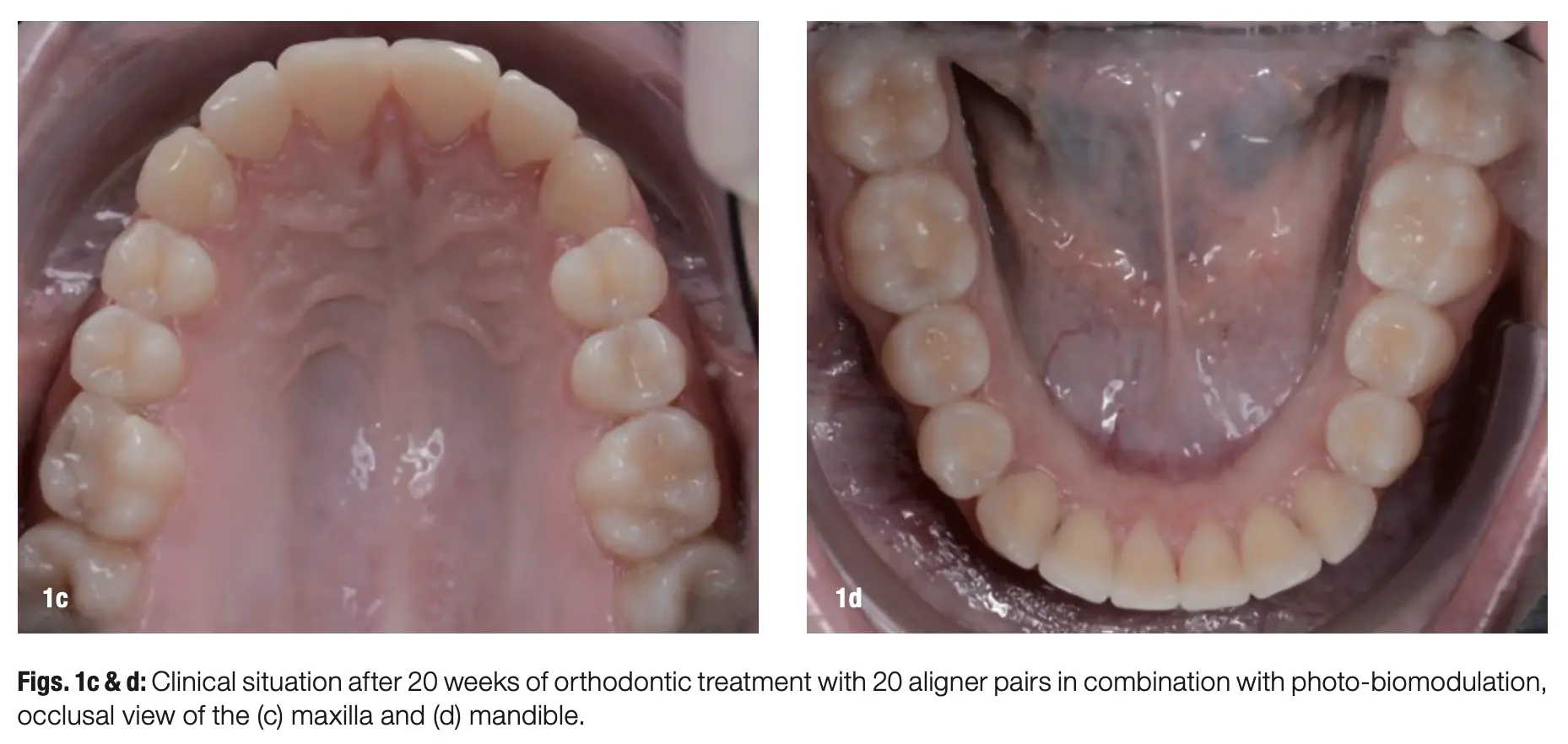
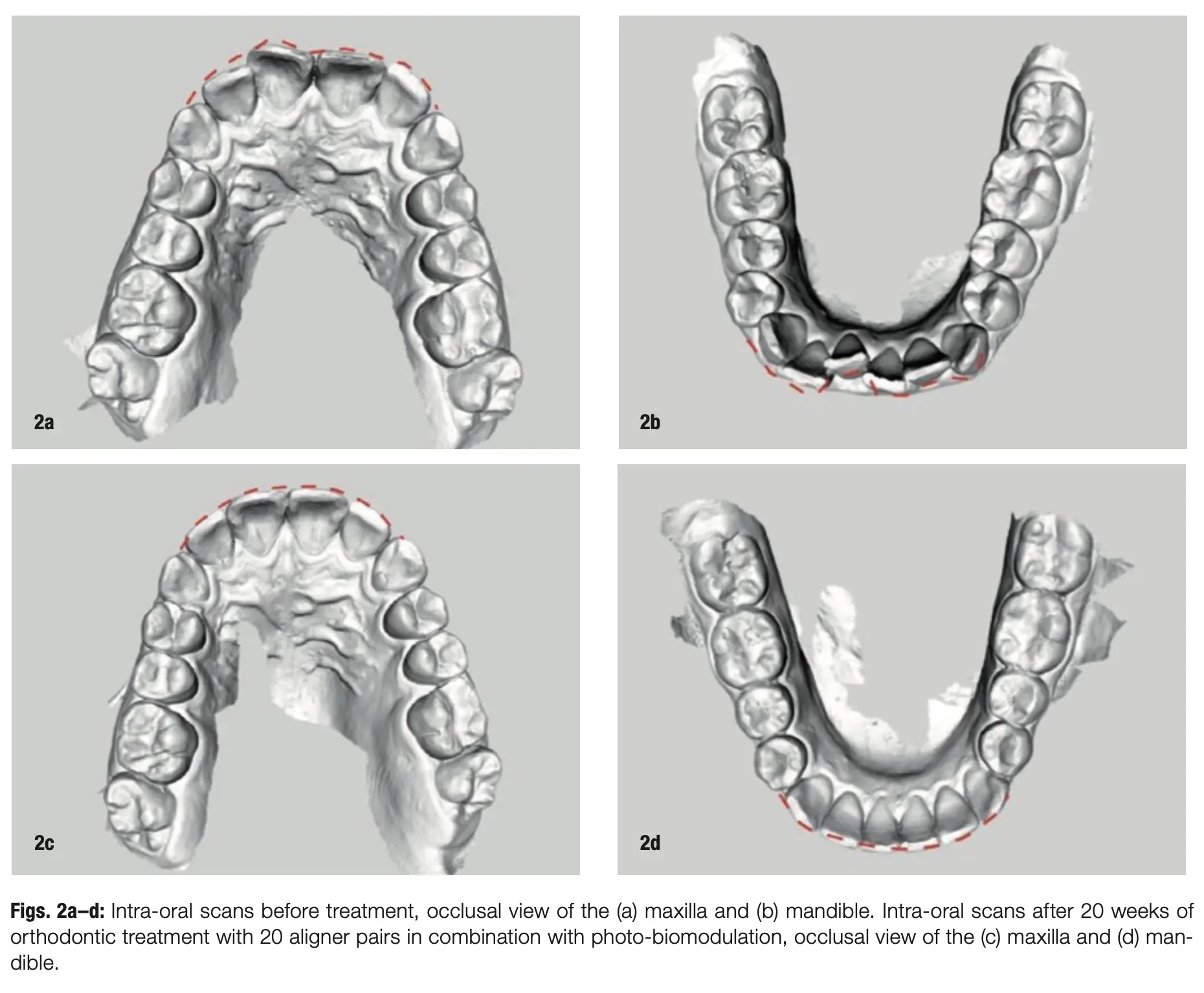
For the past year, we have exclusively been using the ATP38 photo-biomodulation device, and it has been very well accepted in our daily workflow. We are managing to streamline planning for complex cases, from smile design to orthodontic treatment planning and implant placement (when necessary), but what really makes this ecosystem remarkable is the regular combination with the ATP38. We have already had almost ten years of success with this protocol and believe it should be the benchmark in orthodontic therapy, whether with fixed appliances or aligners (Figs. 1–3).
Establishing new protocols is always challenging, especially when including new technology in the workflow. Nevertheless, it is helpful to regard this in light of the metaphor of bamboo growth: it usually takes five years to see growth, so initially it will be difficult to see the real advantages, but as you obtain more cases and see the benefits clinically and receive feedback from patients, it will become clear that all the effort is worth it.
References
1 Impellizzeri A, Horodynski M, Fusco R, Palaia G, Polimeni A, Romeo U, Barbato E, Galluccio G. Photobiomodulation Therapy on Orthodontic Move- ment: Analysis of Preliminary Studies with a New Protocol. Int J Environ Res Public Health. 2020 May 19;17(10):3547. doi: 10.3390/ijerph17103547.
2 Yavagal CM, Matondkar SP, Yavagal PC. Efficacy of Laser Photobiomodulation in Accelerating Ortho- dontic Tooth Movement in Children: A System- atic Review with Meta-analysis. Int J Clin Pediatr Dent. 2021;14(Suppl 1):S94–S100. doi: 10.5005/ jp-journals-10005-1964. PMID: 35082474; PMCID: PMC8754265.
3 Heidari M, Paknejad M, Jamali R, Nokhbatolfoghahaei H, Fekrazad R, Moslemi N, Effect of laser photobiomo- dulation on wound healing and postoperative pain following free gingival graft: A split-mouth triple-blind randomized controlled clinical trial. J Photochem Photobiol B. 2017 Jul:172:109–114.
*Written by Dr. Ana Paz for Aligners Magazine – Dental Tribune.




